Abstract
Introduction: Radioimmunotherapy is a targeted approach to cancer care. It has been shown to improve progression-free survival and quality of life in people with specific types of non-Hodgkin's lymphoma, including CD20- and CD37-positive B-cell lymphoma (Barr et al., 2018 NCT00770224; Green & Press, 2017; Kolstad et al., 2018 NCT01796171; Witzig et al., 2002). It has also been shown to increase the proportion of people who achieve complete response to therapy (Green & Press, 2017). Although CD20-targeted therapy has been approved in the US and Europe for nearly two decades, its use remains limited. At its peak in the UK in 2007, only 57 people with lymphoma were treated with the therapy (Rojas et al., 2019). However, research is ongoing; a PubMed search for 'radioimmunotherapy' and 'lymphoma' produces 1,097 articles in the past 20 years, including over 300 articles in the past ten years. With novel applications (CD37- and CD22-targeted) currently under investigation, it is increasingly important to understand potential system and policy barriers to uptake, such that existing roadblocks are overcome. Tackling these challenges is essential to ensuring that radioimmunotherapy is appropriately integrated into relevant clinical guidelines and care pathways.
Aim: To better understand the policy and system barriers to integration of existing and novel radioimmunotherapy into lymphoma care in the US and the UK.
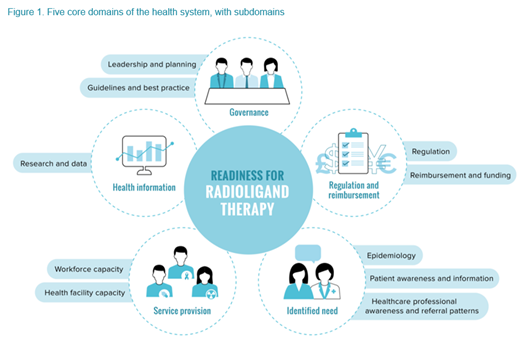
Methodology: We conducted a structured literature review, taking a systems approach, to explore each of the five domains of the health system as outlined in the Radioligand Therapy Readiness Assessment Framework (Figure 1. Five core domains of the health system, with subdomains; The Health Policy Partnership, 2021). This approach allowed us to gain a holistic understanding of what integration of radioimmunotherapy involves and identify potential barriers, from clinical development through to patient care. We also conducted semi-structured interviews with lymphoma experts in the US (N=5) and UK (N=6), including clinicians and nurses ('clinical experts', N=8) and patient advocates ('advocates', N=3). Our work was guided by national expert advisory groups in each country.
Results: While the US and UK health systems are organized and funded very differently, the literature and expert interviews revealed many common strategic challenges to the integration of radioimmunotherapy. These were: 1) low awareness and understanding of radioimmunotherapy among newly licensed healthcare professionals (an issue raised by n=8 clinical experts); 2) limited awareness by patient advocates, patients and policymakers (n=3 advocates); 3) caution around uptake of new radioimmunotherapy agents based on limited access to and use of older treatments (n=7 clinical experts); 4) nonexistent referral pathways and unclear models of working which discourage shared care and hinder multidisciplinary coordination (n=4 clinical experts); 5) lack of recent clinical data and research to support evidence-based use (n=5 clinical experts); 6) reimbursement concerns (n=5 clinical experts).
Policy implications: Taking a systems approach to explore potential barriers to integration of radioimmunotherapy has allowed us to explore potential adaptations needed to achieve multisectoral and multidisciplinary working. Our findings reveal that professional societies, policymakers and patient advocacy groups will need to work together to overcome these barriers by: 1) reaching consensus on timing and eligibility criteria for use of radioimmunotherapy; 2) creating accurate and consistent patient-friendly information; 3) efficiently updating clinical training and treatment guidelines to include approved radioimmunotherapy; 4) developing evidence-based and personalized referral and treatment pathways which ensure consistency of care; and 5) investing in data collection and analysis to continually inform practice.
Morgan: Nordic Nanovector: Consultancy; Advanced Accelerator Applications: Consultancy. Merkel: Advanced Accelerator Applications: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy; Bayer: Consultancy; Bristol-Myers Squibb: Consultancy; Curium: Consultancy; Johnson & Johnson: Consultancy; MSD: Consultancy; Nordic Nanovector: Consultancy; Novartis: Consultancy. Gordon: Zylem Biosciences: Patents & Royalties: Patents, No royalties; Bristol Myers Squibb: Honoraria, Research Funding. Buscombe: Advanced Accelerator Applications Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Wait: Bayer: Consultancy; Curium: Consultancy; Johnson & Johnson: Consultancy; MSD: Consultancy; Advanced Accelerator Applications: Consultancy; Nordic Nanovector: Consultancy; Shionogi: Consultancy; Bristol-Myers Squibb: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy. Dreyling: Genmab: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Astra Zeneca: Consultancy, Speakers Bureau; Bayer HealthCare Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Gilead/Kite: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Abbvie: Research Funding. Mittra: Advanced Accelerator Applications Novartis: Consultancy, Honoraria, Research Funding; Curium: Consultancy, Honoraria; Nordic Nanovector: Research Funding. Gopal: Janssen: Consultancy, Honoraria, Research Funding; Cellectar: Consultancy, Honoraria; Bristol Meyers Squibb: Research Funding; SeaGen: Consultancy, Honoraria, Research Funding; I-Mab bio: Consultancy, Honoraria, Research Funding; Incyte: Honoraria; MorphoSys: Honoraria; Servier: Consultancy, Honoraria; Genetech: Consultancy, Honoraria, Research Funding; IGM Biosciences: Research Funding; Astra-Zeneca: Research Funding; Karyopharm: Consultancy, Honoraria; Takeda: Research Funding; Teva: Research Funding; Agios: Research Funding; Kite: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Acrotech: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; Nurix Inc: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal